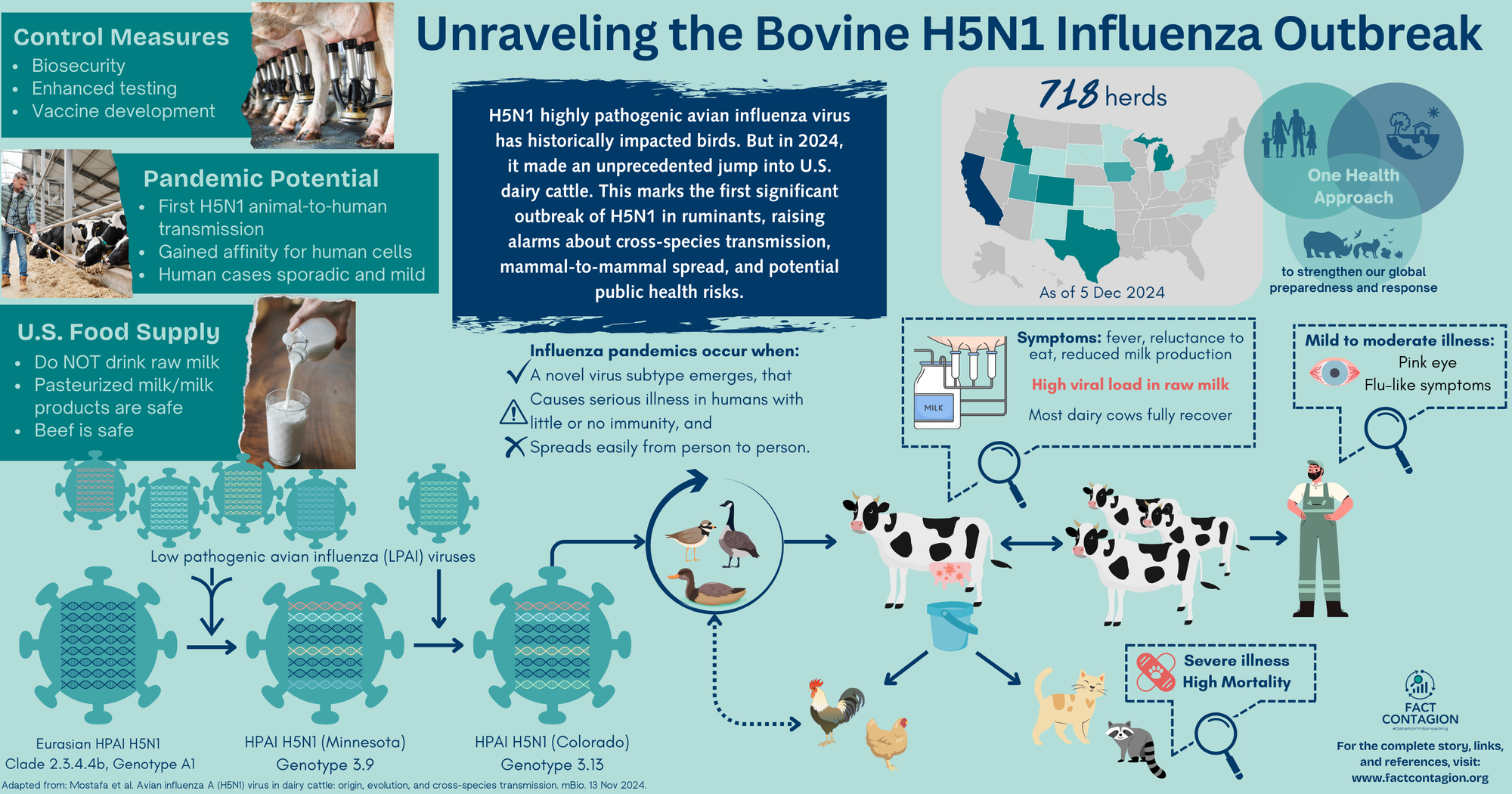
When Cows Catch the Flu: Unraveling the Bovine H5N1 Influenza Outbreak
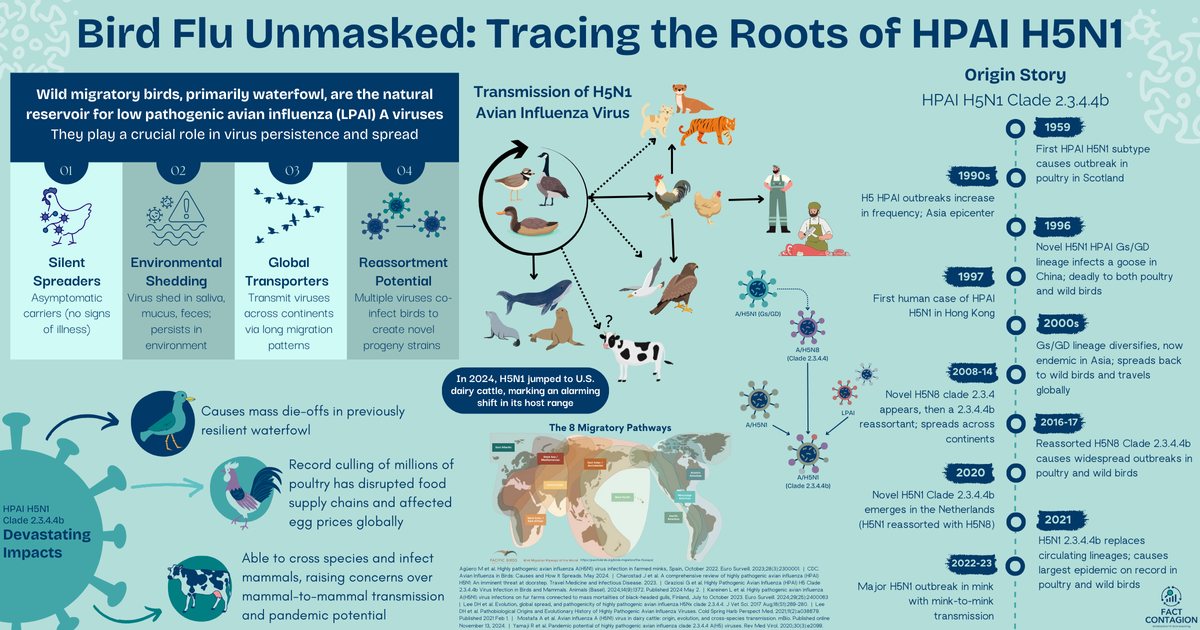
Mountain lions, tigers, and bears - oh my! From house mice and cats, squirrels and foxes, to even dolphins and seals, HPAI H5N1 is a promiscuous little bugger. The list of affected animals is long and continues to grow. Except for mink and marine mammals, HPAI H5N1 does not typically spread animal-to-animal - just sporadic infections that lead nowhere.
Ground Zero
In Feb 2024, large animal veterinarians in the Texas panhandle like Dr. Barb Petersen started receiving calls from dairy farm owners concerned about dead wild birds on their land. Then came the calls about barn cats dying. And dairy cows began showing unusual symptoms: fever, reluctance to eat, and reduced milk production. Routine tests were run. Everything came back negative.
Not much later, in Mar 2024, similar occurrences of sick dairy cows were observed in southwestern Kansas and northeastern New Mexico.
Recognizing the critical nature of the situation, Dr. Petersen sent samples from both barn cats and dairy cows to her friend, Dr. Drew Magstadt, a veterinary diagnostician at Iowa State University. Dr. Gregory Gray, an infectious disease epidemiologist at the University of Texas Medical Branch in Galveston, also began receiving samples from affected farms.
USDA’s National Veterinary Services Laboratory confirmed their answer. Highly pathogenic avian influenza H5N1. A virus never before seen in cattle.
Where did this H5N1 avian virus come from?
The virus belongs to the Eurasian H5N1 lineage goose/Guangdong clade 2.3.4.4b (check my early post for its origin story). This highly virulent clade was first introduced in the US in Dec 2021 from Europe by migratory birds. The virus easily spread into commercial poultry flocks and caused significant morbidity and mortality. Only sporadic infections in wildlife and zoo animals have been reported.
We’ve already established influenza viruses are noncommittal. When replicating, they don’t proof-read their creation, so mistakes (mutations) can easily happen. They also have a segmented genome (like a deck of cards) that can get reshuffled with genetic material from a different influenza virus - creating a brand new hybrid child virus.
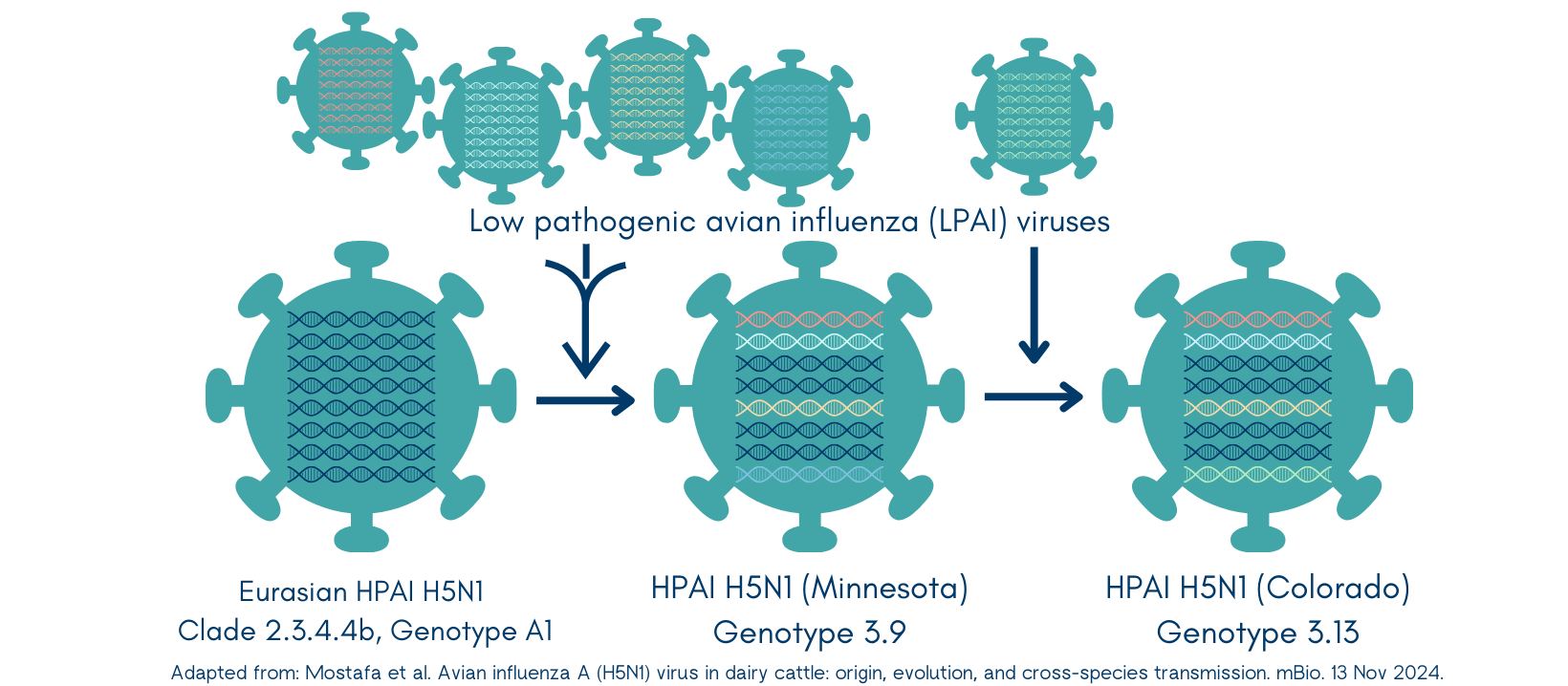
In some not too distant past, this Eurasian wild bird H5N1 virus went through reassortment events (shuffled its cards) with four American non-H5N1 low pathogenic flu viruses. This new virus (genotype 3.9) was first discovered in Nov 2023 in a tundra swan from Minnesota. Also discovered in Nov 2023? Its child (genotype B3.13)! This new virus had again reassorted (replaced one card) with another non-H5N1 low pathogenic virus. Found in a Canada goose in Colorado. Then in another goose from Wyoming in Jan 2024.
Just a few short weeks later, the virus (Clade 2.3.4.4b, Genotype B3.13) spilled over into cattle.
Unusual Thing #1: HPAI H5N1 found a new animal host
Avian flu viruses have an affinity for host cells that express a particular calling sign (a2,3 sialic acids). Human influenza viruses prefer a different calling sign (a2,6 sialic acids). This is an important distinction for virus transmission across species.
Birds express the a2,3 calling sign in their gastrointestinal tract, so this is where the virus invades its avian host. Mammals (including humans) express the a2,6 calling sign in our respiratory tract, hence, where flu usually infects us. (We also have the avian a2,3 calling sign, but it’s deep down in our lower respiratory tract and difficult for the virus to reach.) What about cows? They have both a2,3 and a2,6 calling signs in their mammary glands (and a2,3 in their respiratory tract and cerebrum). And this bovine H5N1 virus? It responds to both calling signs.
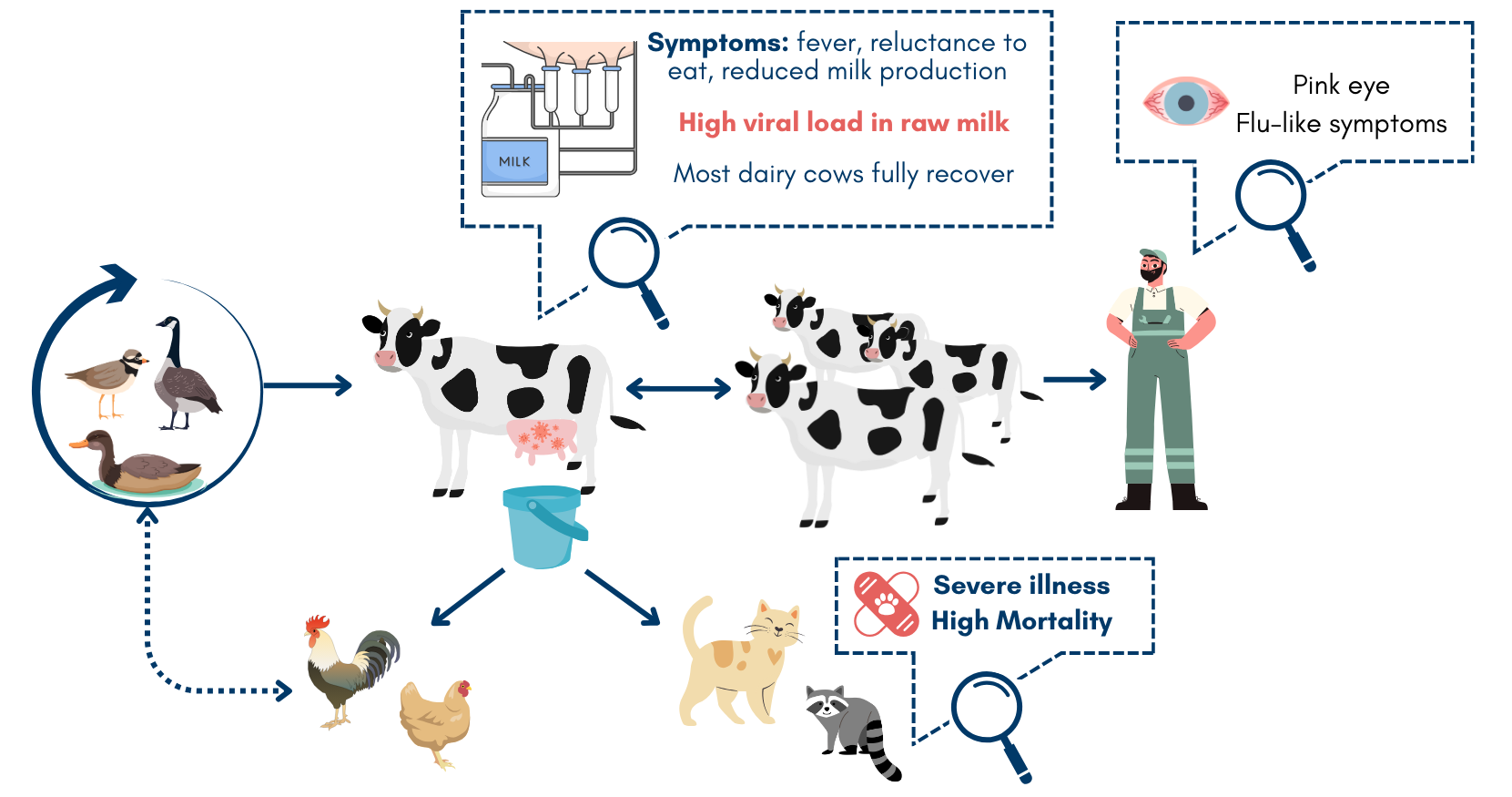
The initial source of virus introduction into the dairy farms is likely to have been either contamination of milking devices or ingestion of contaminated feed via feces from infected migratory birds (the Texas panhandle sits within their Central Flyway).
Unusual Thing #2: The virus began spreading cattle-to-cattle
By May, the USDA had confirmed 69 affected herds across 9 states. The virus was spreading both among and between herds.
While this particular virus is not significantly different from those that affect wild birds and poultry, it gained critical mutations for its adaptation to mammals. Not only is the bovine H5N1 virus able to also respond to the mammalian a2,6 calling sign, the virus also gained the ability to more efficiently exit cells and then spread to other mammals (including humans).
The most likely route of horizontal (cattle-to-cattle) transmission is via direct contact with contaminated milk or milking equipment. There is a particularly high viral load in the milk of infected cows. Barn cats were likely fed contaminated raw milk. There is little evidence to suggest the virus is spread via the respiratory route.
Interstate cattle movement then allowed the virus to swiftly spread between herds. APHIS estimates that ~1,400 lactating dairy cows are moved across the US every week!
Other animals on affected farms are also being infected, and in turn providing a second source of virus transmission to other animals, as well as people. In cats, the virus is causing severe morbidity and high mortality. The virus has also been found at nearby poultry facilities.
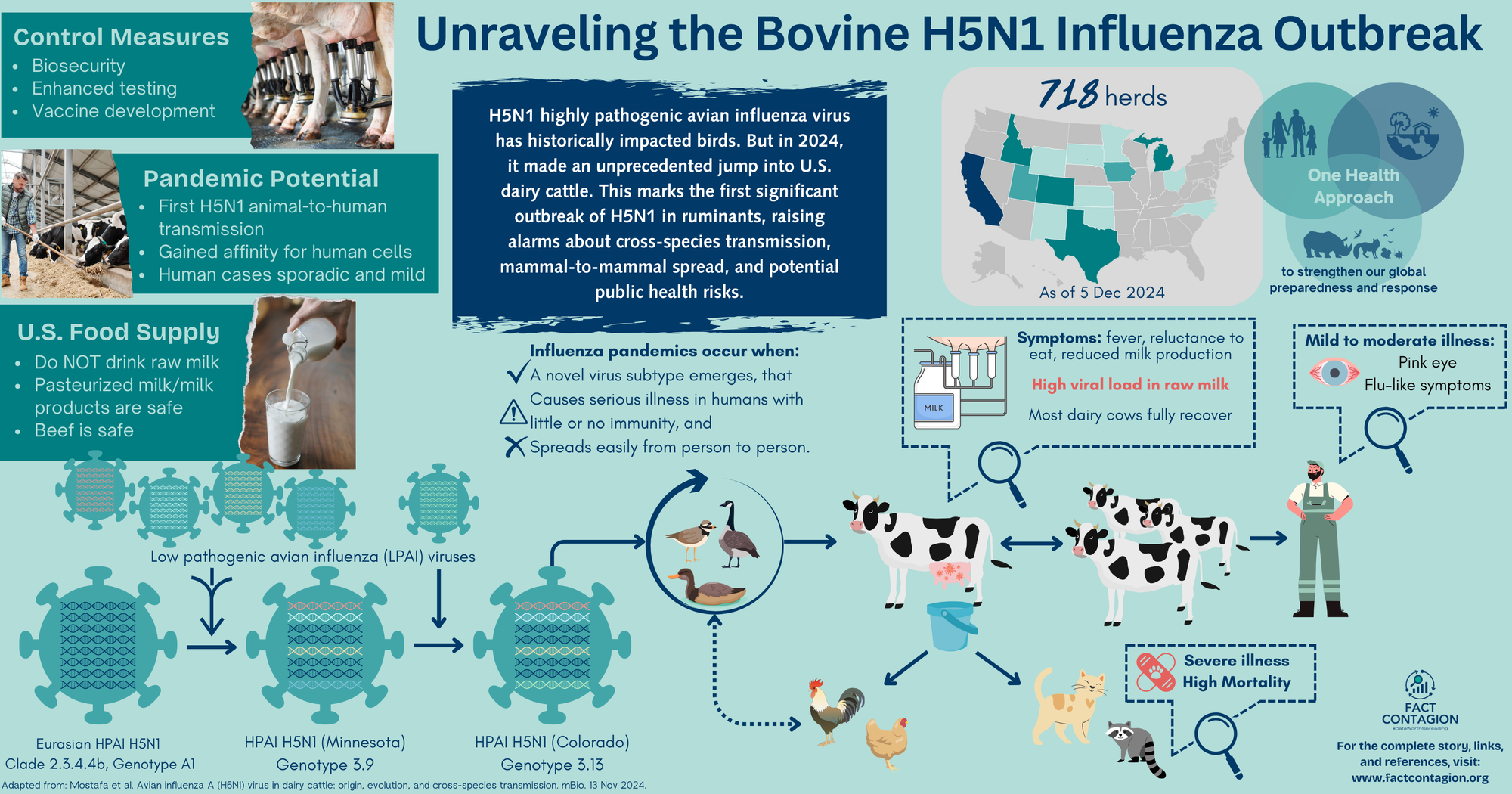
Current Situation in Cattle Herds
As of 5 Dec 2024, 718 affected dairy herds have been confirmed across 15 states. In the past 30 days, almost all cases have been in California across 269 herds (4 additional herds in Utah). Most cattle fully recover with supportive therapy; the death/culling rate is <2%. However, there are likely many more affected cows than what is being reported to USDA. A key reason being that farmers may be reluctant to test due to remediation requirements.
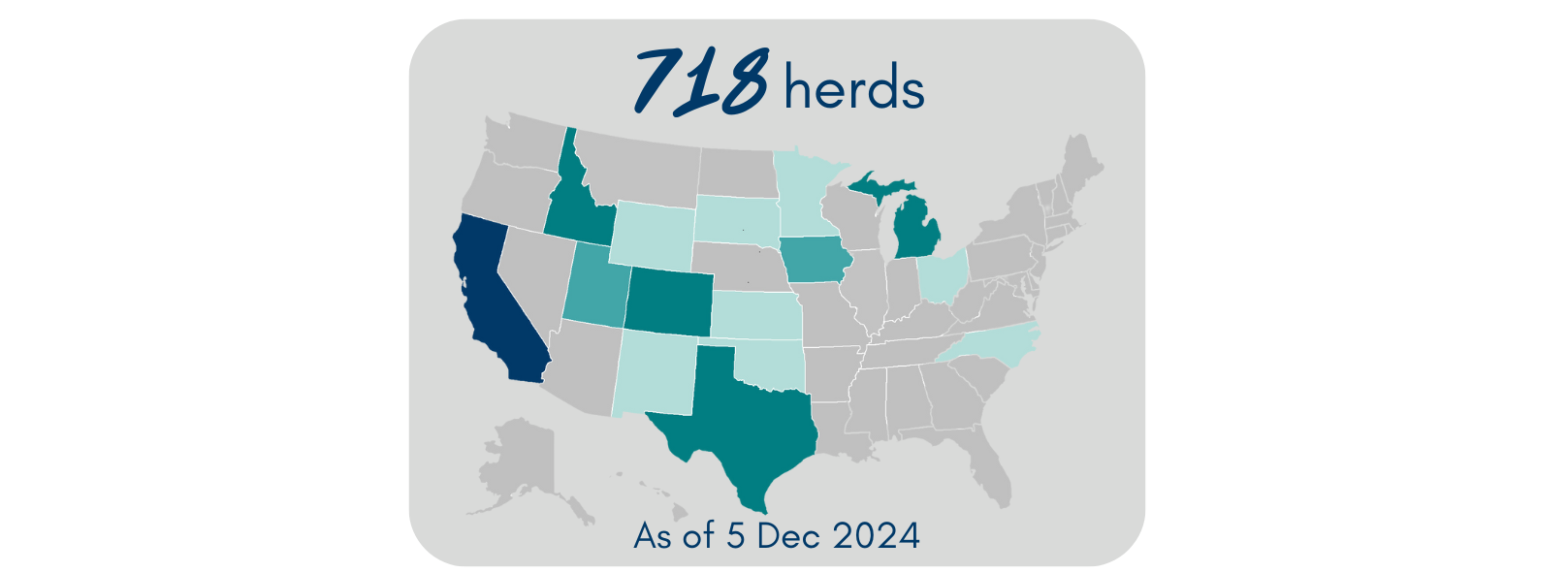
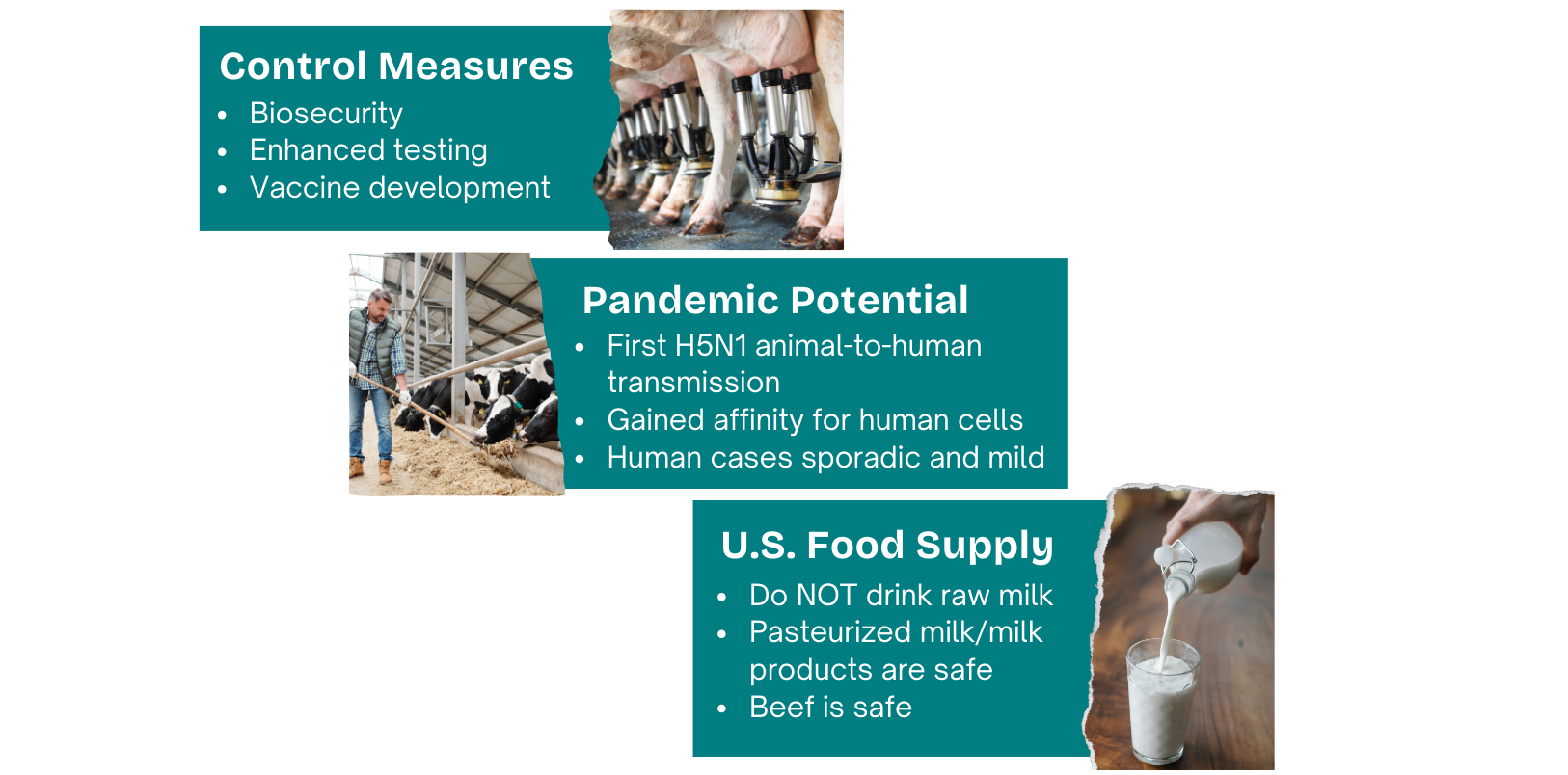
Veterinarians and farmers are encouraged to practice good biosecurity. That includes actively monitoring for and separating sick animals, testing, minimizing cattle movements, and isolating new received dairy cattle. Positive cases must be reported to USDA’s Animal and Plant Health Inspection Service (APHIS). USDA also enacted a federal order requiring all lactating dairy cattle be tested for H5N1 prior to interstate movement. States have enacted further importation restrictions.
Zoonotic transmission to animal workers
Not long after H5N1 showed up in cows, the CDC confirmed the first human infection on Apr 1 in a person with exposure to sick dairy cows in Texas. This is the first documented mammal to human transmission of HPAI H5N1.
Since then, 55 human cases of H5N1 have been detected through targeted surveillance of ~9000 people exposed to infected animals, of which ~400 were tested. In Nov, CDC confirmed the first reported case in a child, who resides in California; the source of exposure is still being determined.
Symptoms have generally been mild to moderate, with conjunctivitis (pink eye) commonly reported, as well as flu-like respiratory symptoms. Clinical presentation varies depending on duration and route of exposure, and individual health status.
However, we’re essentially flying blind here. Just as producers are reluctant to test/report sick animals, there is significant distrust in the farming community regarding divulging workers' personal information to health officials, the implications to their herds, and simply lack of access to healthcare. In addition to underreporting of cases, there may also be undetected asymptomatic infections.
So what does this mean for milk and dairy products?
Point #1: Pasteurized milk and dairy products are 100% safe.
Pasteurization is simply a food preservation technique that kills harmful bacteria (and thus extends shelf life) using heat. Milk is heated to a specific temperature for a given time (eg, 161F for 15 seconds). No chemicals or other additives involved. Pasteurization inactivates the H5N1 virus, and milk from impacted animals (albeit those that are reported) is being diverted or destroyed; therefore, commercially available pasteurized milk is safe for consumption.
FDA is testing retail dairy samples from multiple states, including pasteurized milk and milk products (eg, butter, ice cream, cheese) as well as aged raw milk cheese. Virus fragments were found in some samples, but there was no live, infectious virus present. Pasteurization is effective in inactivating the virus.
Point #2: Raw milk and raw milk products are NOT considered to be safe (ever, but especially now).
This virus is thriving in dairy cows’ udders, and very high levels of live virus is shed in the raw milk from infected cows. This fact is fueling the spread among cattle herds. It is putting other animals, and humans, at risk if they ingest contaminated raw milk. (In a 2019 study, 4.4% of US adults reported consuming raw milk at least once in the previous year; of which 1.6% consumed it at least once a month, and 1% consumed it at least once per week. I’m going to assume that this has only increased, 5 years later.)
Viable H5N1 virus has been detected in a retail raw milk product purchased at a store in California, for which a recall was issued. No human illnesses were reported in connection.
Let’s all say it together: CONSUMING RAW MILK OR RAW MILK PRODUCTS SHOULD BE AVOIDED - always. Any perceived benefits cannot and will never outweigh the risks.
And what about beef?
USDA’s Food Safety and Inspection Service (FSIS) is testing retail ground beef in states with affected cattle herds. Nothing has been detected - no virus particles, let alone viable virus. The meat supply is considered safe. As always, it’s recommended to cook beef to an internal temp of at least 165F (including meat you feed your pets).
Do we even have a good handle on the situation?
So if reported human and animal cases are based on voluntary testing and reporting, can we even have a good handle on the current situation?
My gut answer: nope.
The CDC is leveraging several year-round surveillance systems to monitor key flu indicators, but they don’t provide a complete picture. One of those is monitoring our wastewater. For the week ending Nov 23, 50 out of 310 sites (16.1%) reported detection of H5 virus particles. All but two sites are located in California, corresponding with the majority of recent bovine cases. The source is likely contaminated dairy milk, including anything disposed of by consumers.
What about flu vaccines?
The seasonal flu shot does not protect against H5N1. But YES, you should still be getting it to reduce viral spread and severity if infected with seasonal flu. It’s especially critical that agricultural workers in contact with animals receive seasonal flu shots. Workers are a source of human influenza viruses that can co-infect poultry and other animals. They are also at risk of co-infections with human and avian influenza viruses at the same time. Both of these situations are giving flu viruses unnecessary opportunities to reassort (shuffle their cards) and produce new zoonotic viruses with pandemic potential.
Vaccines targeting the H5N1 flu subtype
Poultry: Several HPAI H5 vaccines for poultry exist. Historically, they haven’t been readily used in the US and other countries due to trade barriers (we rely on biosecurity and culling). And they aren’t a good match for the new Clade 2.3.4.4b virus. As of 2023, the USDA began new trials in poultry to protect against this new variant.
Cattle: USDA recently began two vaccine field trials for dairy cattle, and they may expand into other species too.
Humans: If the bovine H5N1 virus starts spreading in humans, the US has the ability to rapidly develop and deploy a H5-targeted vaccine. We have a stockpile of a vaccine against avian H5N1 flu, but it’d need to undergo more testing in humans before it could be released under an Emergency Use Authorization. (Or they may decide to first update it to more closely match the circulating strain.) The CDC also has the capabilities to produce a H5 Clade 2.3.4.4b human flu vaccine using candidate vaccine viruses. Lastly, novel platforms like mRNA vaccines could be rapidly developed by researchers.
How does all of this impact you? Should we be worried?
This bovine H5N1 virus has gained the ability to spread across mammalian species, including humans - a significant development. So far, evidence suggests only sporadic human cases - no instances of human-to-human transmission.
In an experimental study of ferrets, the bovine H5N1 virus isolated from an infected person spread efficiently between ferrets via direct contact (in the same enclosure), but it did not efficiently spread between ferrets via respiratory droplets (in adjacent enclosures; 1 of 3 ferrets infected, 33%). These are common “animal model” studies done for flu viruses to better understand transmission dynamics, as well as infection patterns, disease severity, and immune response.
Therefore, people in close or prolonged, unprotected contact with infected animals or their environments are considered at moderately-high increased risk. Those in routine contact with cattle or wild birds in general remain at a slightly elevated risk.
In our current state, the risk to the general public is LOW.
- Tips for prevention
- A helpful resource from American Veterinary Medical Association (AVMA) tracking the situation
But (and this is a big but), this bovine H5N1 responds to both avian (a2,3) and human (a2,6) calling signs. It’s shown an ability to infect a wide range of species. And a recent report revealed a single point mutation found in the bovine H5N1 virus isolated from an infected person has made the switch from preferring the avian calling sign to human’s a2,6.
There is certainly concern that through a few more mutations, the virus could evolve and adapt to efficiently spread human-to-human. Just like we’ve heard with COVID - the more infections (in both animals and humans; even asymptomatic), the more opportunities for these mutations to happen.
Preventing the next pandemic
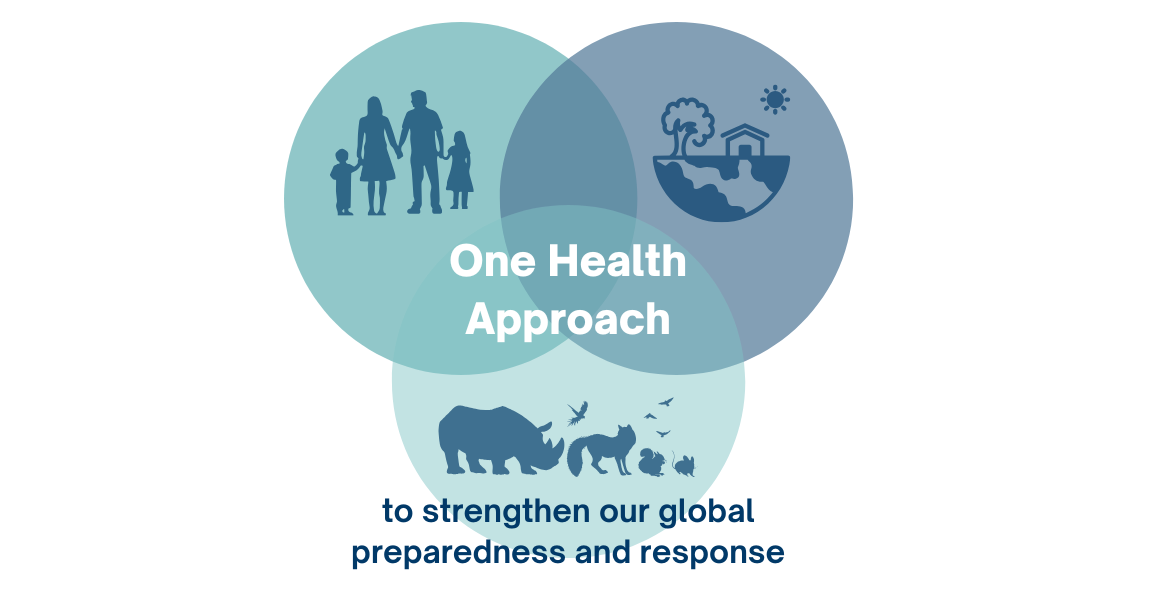
The number of human cases remains low. However, the ability for the H5N1 virus to spread among mammals is a transformative shift and increases its pandemic potential. This highlights the need for a One Health, interconnected approach across human, animal, and environmental health disciplines to strengthen our global preparedness and response.
Science is not red or blue, and no administration has adequately responded to emerging infectious disease threats, but I’d be remiss to not at least mention the significant concerns about the incoming administration, especially with raw milk proponent RFK Jr at the helm of Health & Humans Services (with jurisdiction over NIH, CDC, and FDA). Both outgoing and incoming administrations must commit to effective surveillance and research activities to track the virus's evolution and evaluate its potential public health risks. Commit to enhanced, or even mandated, testing of cows, milk, and farm workers. Commit to making preparations for the H5 vaccines. Commit to evidence-based decisions, improved transparency and effective communication.
What can you do?
Stay vigilant. Stay informed. Participate in the conversation. Ask questions. This story will continue to unfold. The more we know, the better we can protect ourselves.
If you’d like a Primer that introduces you to zoonotic influenza virus biology and transmission, check out this post:
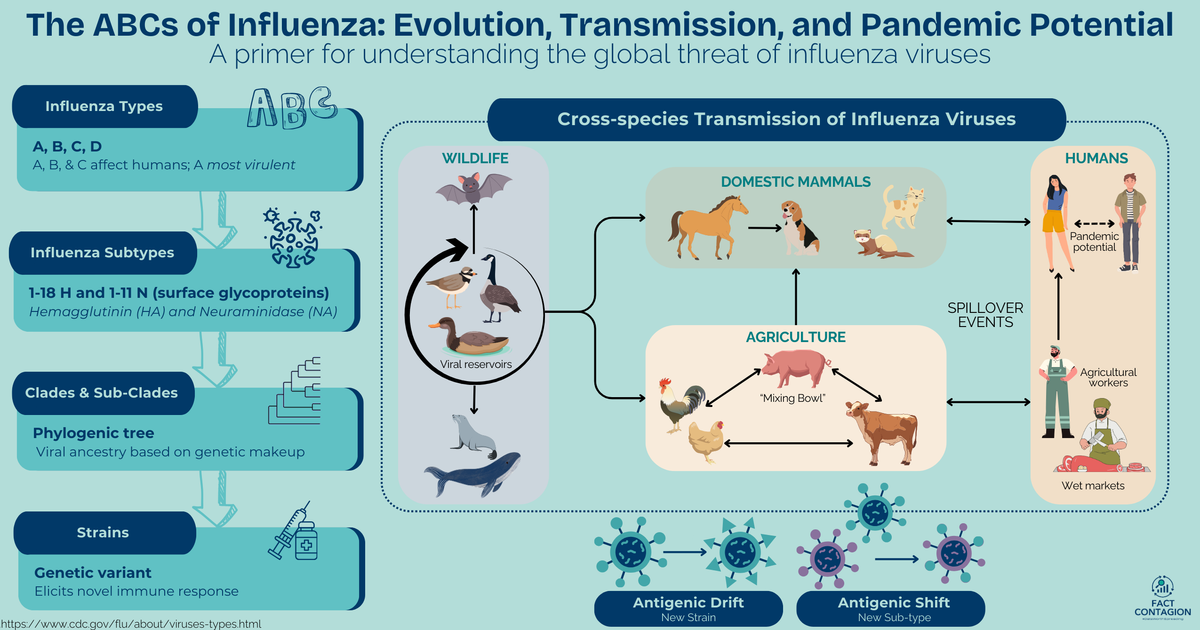
If you’d like to learn the history of the avian influenza virus (HPAI H5N1 Clade 2.3.4.4b) causing the current outbreak in US dairy cows, check out this post: